By Yifan Zhang
Renewable energy is becoming increasingly relevant in today’s world, and finding sustainable energy sources is essential to keep up with our world’s high energy demand while simultaneously preserving our planet and its resources for future generations.
Dye sensitized solar cells (DSSCs) are a possible long-term solution to this pressing issue, as they have a low production cost, relatively simple preparation process, and abundance of materials. However, DSSCs also have drawbacks: they are less stable long-term due to innate characteristics of dyes and are much less efficient compared to other energy sources. While solar panels have a larger upfront cost, they are more durable and can convert more sunlight into energy than DSSCs. Still, there is a lot of potential for DSSCs to become more widespread, and efforts are being made to increase their efficiency and longevity.
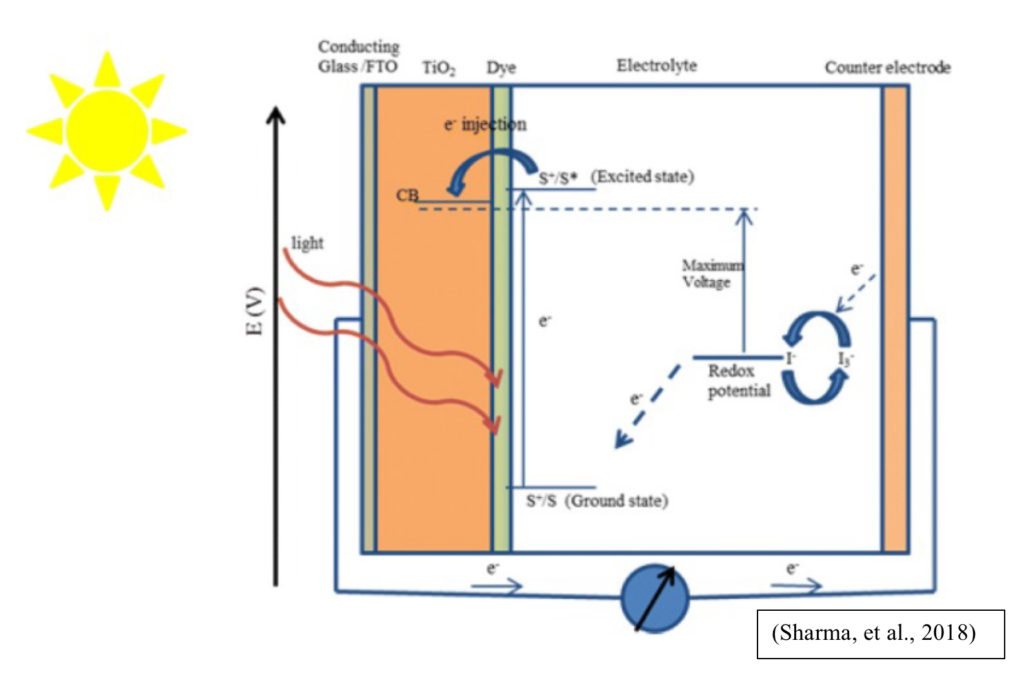
These solar cells are made of four key parts; the electrode, electrolyte, dye, and counter electrode. The electrode is coated in the dye, while the counter electrode is coated in an electrolyte solution. By placing the electrode and counter electrode against each other, this allows electrons to flow freely between the two.
DSSCs were first constructed using chlorophyll from spinach in 1972 and worked by having the dye molecules, which were excited from exposure to sunlight, undergo electron injection (the electrons go from the dye to the semiconductor nanoparticles), turning photons into electricity (Sharma, et al., 2018). This cell was not efficient, however, and in order to increase the efficacy, UC Berkley researchers Brian O’Regan and Michael Grätzel focused on improving how much the dye could coat the cells. Instead of using a zinc oxide coated electrode, titanium dioxide (TiO2) was used instead, which allowed more dye to adhere to the TiO2. Because there was more dye on the electrode, more sunlight was able to be absorbed, improving efficiency of harvesting light from 1% to 7%. The titanium dioxide coated cells are referred to as Grätzel cells, and these DSSCs are typically constructed with fluorine-doped tin oxide (FTO) or indium-doped tin oxide (ITO) glass and annealed with TiO2. The glass serves as the electrode, and its low opacity allows more sunlight to pass through and excite the dye molecules.
When the cell is soaked in dye, the dye molecules covalently bond to the TiO2, increasing light absorption. The ideal dye should be luminescent, cover the UV and NIR spectrum, and be hydrophobic to improve cell stability, as the dye is less likely to be distorted by water. While dyes sourced from plants are cheap, they do not have these properties, and synthetic dyes have been found to be a better alternative (Tontapha, et al., 2021).
The electrolyte should be stable long-term, be able to efficiently regenerate the oxidized dye, and be non-corrosive. I-/I-3 is efficient but is not stable long-term and can cause the dye to detach from the TiO2. A T2/T- electrolyte has also been used, because of its low corrosiveness and long-term stability, but I-/I-3 is more common.
DSSCs have yet to become as efficient as silicon solar cells, but if we invest resources into developing stable dyes and electrolytes and continue to improve how well the dye is able to bond to the electrolyte, DSSCs have the potential to be a cheaper, more sustainable alternative to traditional energy sources.
Bibliography:
Johnson, J., & Chasteen, S. (2006). Solar Cells: Juice From Juice. Cornell
Center for Materials Research.
Keith. (n.d.). How to Build and Use a Dye-Sensitized Solar Cell (DSSC). Autodesk
Instructables.
Sharma, K., Sharma, V., & Sharma, S.S. (2018). Dye-Sensitized Solar Cells:
Fundamentals and Current Status. Springer Nature, 13.
Tontapha, S., Uppachai, P., & Amornkitbamrung, V. (2021). Fabrication of
Functional Materials for Dye-sensitized Solar Cells. Frontiers, 9.
