Enthalpy and Calorimetry Overview
By Yifan Zhang
Calorimetry measures the amount of heat produced in a reaction. Similar to the word calorie, which is a value used to measure the amount of energy in a serving of food, calorimetry can be used to determine the change in heat, which can help determine the change in enthalpy. Enthalpy (H) is the internal energy of the system (i.e., the water and chemicals) plus the product of pressure and volume, and is a state function, which means that its value does not depend on the path taken to reach the current state. Additionally, enthalpy is an extensive property, as it depends on the amount of substance in the reaction. Enthalpy also depends on the states of the reactants and products. For example, going from a solid to a liquid and going from a liquid to a gas requires energy to break the bonds in the substance (the opposite is true for the reverse processes), so the state of the reactants would influence enthalpy (Brown, 2012).
The change in enthalpy (∆H) measures heat flow between the system, the portion singled out for study, and the surroundings, everything else besides the system, and is represented by the equation ∆H= ∆E + P∆V. Energy is the capacity to do work or transfer heat. Work is defined as the energy used to cause an object to move against a force, and the heat added to or leaving the system is represented by q. It should be noted that energy is always conserved, as stated in the first law of thermodynamics (Hurley & Shamich, 2015). For example, when the system gains heat, the surroundings loose heat, and when the system loses heat, the surroundings gain heat.
The heat of solution (∆Hsoln) is derived from the equation ∆H = qP where qP is the heat gained or lost at a constant pressure. This would refer to the contents of a calorimeter if the reactants were in a solution. The heat flow can be derived from the equation qsoln = -Cs x m x ∆T, where Cs is the specific heat of the solution, m is the grams of the solution, and ∆T is the change in temperature of the water that the reaction takes place in.
It is also important to note that qsoln = -qrxn, because the heat flow between the reaction and solution are opposite to each other. If the reaction gains heat, the surroundings loose heat, and vice versa. When qrxn is negative, that indicates the reaction is exothermic and the temperature of the solution in the calorimeter increases, and when qrxn is positive, that indicates the reaction is endothermic and the temperature of the solution in the calorimeter will decrease. If the dissolution of a chemical is an endothermic process, the reaction needs heat, so the temperature of the solution will drop and it will have a positive ∆Hsoln (Brown, 2012).
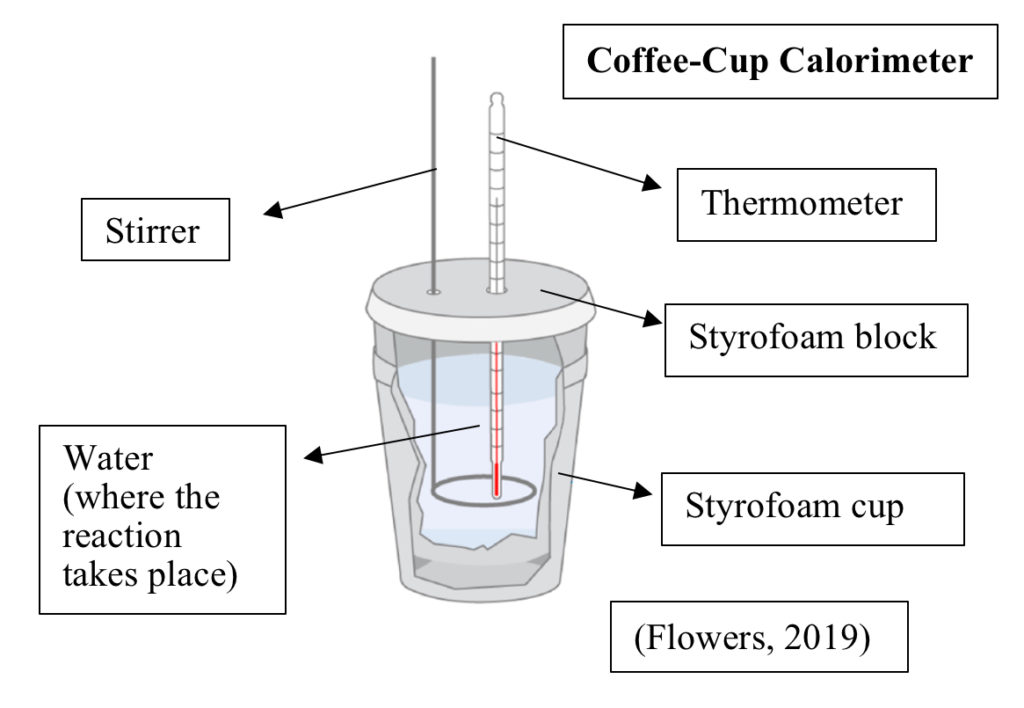
In order to measure qsoln, a calorimeter is used. It’s possible to do this at home using a coffee-cup calorimeter. A coffee-cup calorimeter is composed of a styrofoam cup with a styrofoam block on top, acting as an insulated stopper to prevent heat from entering or escaping the calorimeter. The styrofoam block contains two holes for a thermometer and a stirrer (Flowers, 2019). The styrofoam cup and block both prevent heat from the lab surroundings (in the air, the table, etc.) from being transferred to the system.
The dissolving of certain compounds is also a spontaneous process, and like all spontaneous reactions, it adheres to the second law of thermodynamics. For spontaneous processes, the entropy (S) of the universe is always increasing and can be represented by the equation ∆Suniv = ∆Ssystem + ∆Ssurroundings > 0. Entropy in an individual system can decrease, but it requires an input of energy. Additionally, the second law of thermodynamics also states that for reversible processes, the entropy of the universe stays the same, and can be represented by the equation ∆Suniv = ∆Ssystem + ∆Ssurroundings = 0.
Enthalpy is an important concept that can be applied to everyday life, such as calorie measurement and the natural dissipation of energy throughout the universe. It’s the cornerstone of thermodynamics and understanding how energy flows between systems allows us to further grasp how molecules behave.
Bibliography:
Brown, T. L. (2012). Chemistry, the central science, AP edition (12th ed.).
Pearson Education/Prentice Hall.
Flowers, P., Theopold, K., Langley, R., & Robinson, W. (2019, February 14).
Chemistry 2e. OpenStax. Retrieved July 22, 2025, from
https://pressbooks.bccampus.ca/chemistry2eengineering/front-matter/cover/
Hurley, K., & Shamieh, J. (2015). Enthalpy. LibreTexts Chemistry. Retrieved July
22, 2025, from https://chem.libretexts.org/Bookshelves/
Physical_and_Theoretical_Chemistry_Textbook_Maps/
Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Thermodynamics/
Energies_and_Potentials/Enthalpy
