Polymer Synthesis: New Mechanistic Insights and Catalytic Control
By Allison Tang
Polymer synthesis is the procedure through which long-chain
macromolecules—polymers—are assembled from small monomer building blocks. As outlined
in Vollhardt & Schore’s Organic Chemistry (2018), polymers are largely produced by addition
polymerization of alkenes, where monomer units are successively added, converting double
bonds into single-bonded backbones. This procedure of molecular assembly forms the basis for
most everyday materials, such as high-strength fibers and biomedical devices.
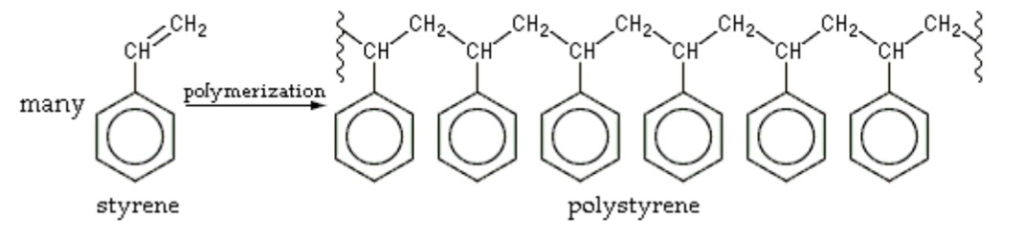
There are two main methods of polymer synthesis: step-growth polymerization and chain-growth
polymerization (addition polymerization) (Reimschuessel, 1975). Chain-growth polymerization
is initiated by a reactive species that activates a monomer. Once activated, new monomers add to
the propagating chain rapidly, one by one. Chain-growth polymerization is widely used for
synthesizing commercially important plastics like polyethylene and polystyrene (National
Polymer, 2024). The efficiency of chain-growth polymerization makes it an excellent choice for
synthesizing polymers with very high molecular weights (Reimschuessel, 1975).
Step-growth polymerization (condensation polymerization) employs monomers with two or
more reactive groups—such as diols, diamines, and diacids—that build up in a series of steps.
Each reaction typically releases a small by-product like water or HCl. Unlike chain-growth, step-
growth polymerization does not require an initiator but relies on the functional groups present on
the monomers. Step-growth polymerization is commonly used in the preparation of nylons,
polyesters, and epoxy resins (Sigma-Aldrich, n.d.).
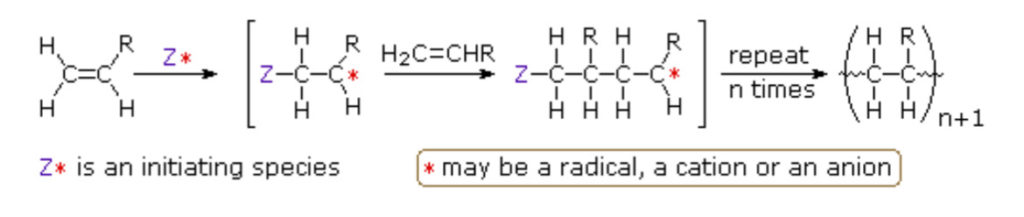
Conventional polymerization often yields polymers with broad molecular weight distributions
and irregular architectures. However, controlled/living polymerization methods enable chemists
to precisely manipulate polymer structures. An example is Atom Transfer Radical
Polymerization (ATRP), which uses metal catalysts—most notably copper complexes—to
reversibly activate and deactivate propagating chains, ensuring accurate control over molecular
weight and architecture (Sigma-Aldrich, n.d.). Reversible Addition-Fragmentation Chain
Transfer (RAFT) polymerization employs chain-transfer agents to regulate chain growth,
allowing the formation of block copolymers and complex architectures like stars and brushes
(Chiefari et al., 1998). These methods enable scientists to design polymers with predictable
lengths, narrow molecular weight distributions, and specialized shapes.
The arrangement of monomer units in a polymer chain significantly affects its physical
properties. Specific catalysts are employed to manage this arrangement. Ziegler-Natta
catalysts revolutionized polymer synthesis by enabling the production of polymers like
polypropylene with well-ordered isotactic or syndiotactic morphologies, resulting in enhanced
strength and crystallinity (Vollhardt & Schore, 2018). Metallocene catalysts offer even greater
control by using single-site catalysts that precisely regulate polymer chain growth, achieving
uniform molecular weights and superior material properties (National Polymer, 2024).
Additionally, Ring-Opening Metathesis Polymerization (ROMP) opens strained cyclic
monomers and links them into unsaturated polymer chains, a technique used to create synthetic
rubbers and functional polymer networks with tunable mechanical properties (Sigma-Aldrich,
n.d.).
With rising environmental concerns, polymer chemists are focusing on sustainable
alternatives. Polylactic Acid (PLA), derived from renewable resources like corn starch or
sugarcane, is a biodegradable and compostable polymer being marketed as an eco-friendly
substitute for petroleum-based plastics (National Polymer, 2024). Furthermore, sequence-
controlled polymers are synthetic macromolecules built with precisely arranged monomer
sequences. By placing monomers at specific positions on a molecular level, scientists can design
materials with highly specialized functions, from molecular recognition to programmable self-
assembly (Sigma-Aldrich, n.d.).
In conclusion, polymer synthesis is a rapidly evolving field that fuses chemistry and materials
science. From foundational chain-growth and step-growth mechanisms to advanced controlled
polymerization techniques and catalyst designs, scientists now have unprecedented control over
polymer structure, function, and environmental impact (Reimschuessel, 1975; Vollhardt &
Schore, 2018).
Bibliography:
Chiefari, J., Chong, Y. K., Ercole, F., Krstina, J., Jeffery, J., Le, T. P. T., Mayadunne, R. T. A.,
Meijs, G. F., Moad, C. L., Moad, G., Rizzardo, E., & Thang, S. H. (1998). Living free-radical
polymerization by reversible addition–fragmentation chain transfer: The RAFT
process. Macromolecules, 31(16), 5559–5562. https://doi.org/10.1021/ma9804951
National Polymer. (2024, April 18). Synthesis of polymers and resins. National Polymer.
Retrieved August 4, 2025, from https://nationalpolymer.com/blog/synthesis-of-polymers-and-
resins/
Reimschuessel, H. K. (1975). General aspects in polymer synthesis. Environmental Health
Perspectives, 11, 9–20. https://doi.org/10.1289/ehp.75119
Sigma-Aldrich. (n.d.). Polymer synthesis and analysis. Merck KGaA, Darmstadt, Germany.
Retrieved August 4, 2025, from https://www.sigmaaldrich.com/US/en/applications/materials-
science-and-engineering/polymer-synthesis
Vollhardt, K. P. C., & Schore, N. E. (2018). Organic chemistry: Structure and function (7th ed.).
W. H. Freeman. Retrieved
from https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry
_(Vollhardt_and_Schore)/12%3A_Reactions_to_Alkenes/12.15%3A_Synthesis_of__Polymers
