By Yifan Zhang
Light brings life to the world—nature and humans revolve around the sun. The sun’s rays cast their glow on half of the Earth, exposing us to the full electromagnetic spectrum of infrared, visible light, and ultraviolet radiation. The electromagnetic spectrum is the range of energy, emitted in the form of radiation, that travels in wave form. These waves, of which visible light is a part, are composed of tiny, massless particles called photons that carry electromagnetic energy and exist at the quantum scale. A longer wavelength (the distance between two crests of a wave, or in other words, how long a segment of the wave is before it repeats again) indicates lower energy, while a shorter wavelength indicates higher energy.
Photons exhibit characteristics of both waves and particles, as postulated by Einstein in the early twentieth century, a fundamental discovery in the field of quantum mechanics. Isaac Newton believed light to be a particle because it could bounce off objects, and Max Planck supported this idea with his work in black-body radiation (U.S. Department of Energy, n.d). But the double-slit experiment proved that light behaves like a wave, and, when observed, behaves like a particle.
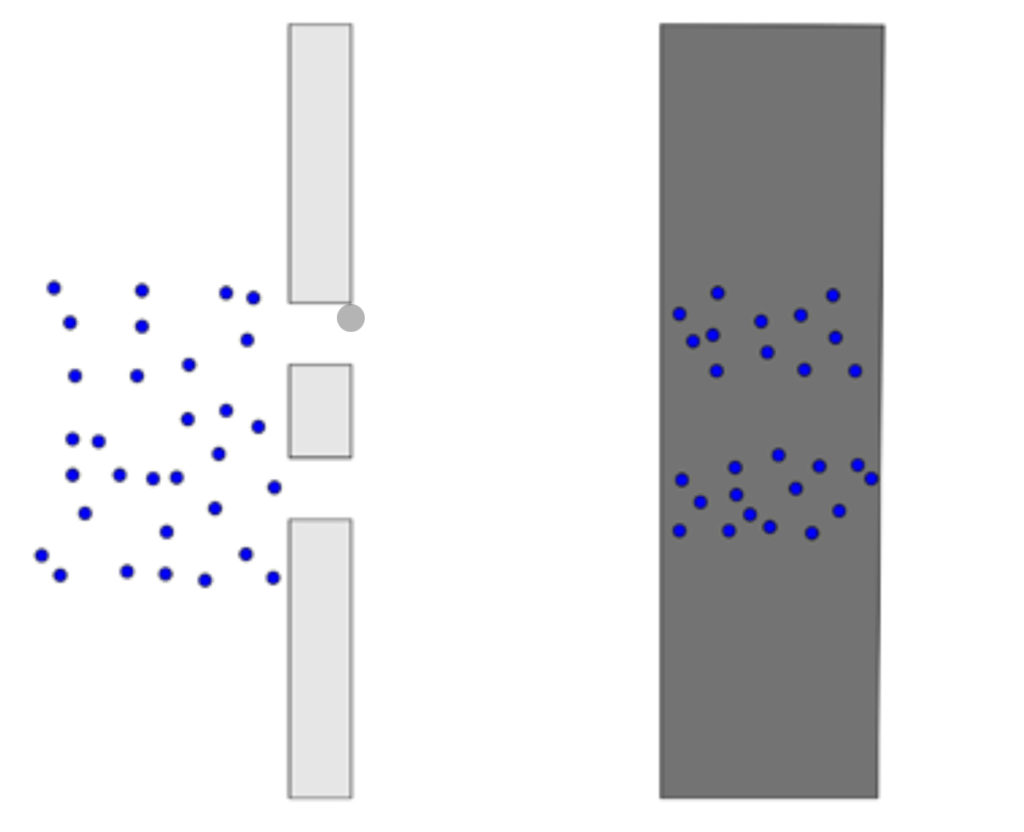
Imagine a wall with two slits in it and a solid wall just a few feet behind it. And perhaps you throw a hundred balls at random towards the wall. The balls that hit the back wall had to have passed through the slit, the area of the solid wall that has been hit by the balls thrown will be the same width as the slits. This behavior applies to photons, and the more photons that hit an area, the area where the photons hit the solid wall will be brighter since there are more photons there (Thomas & Freiberger, 2020).